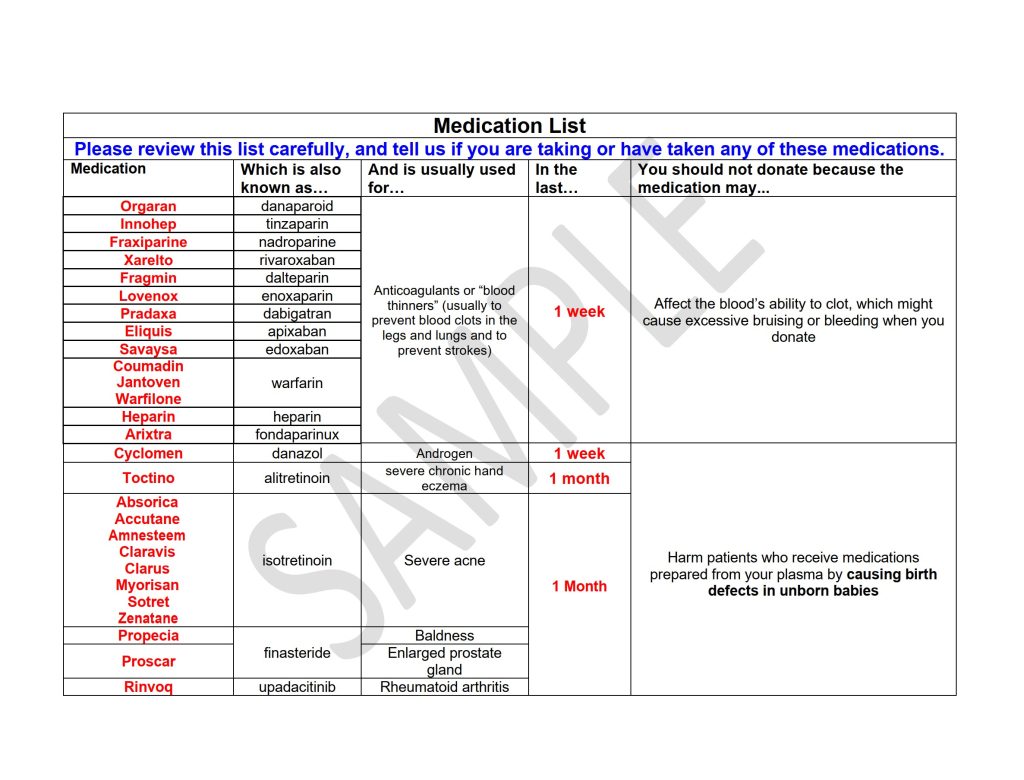
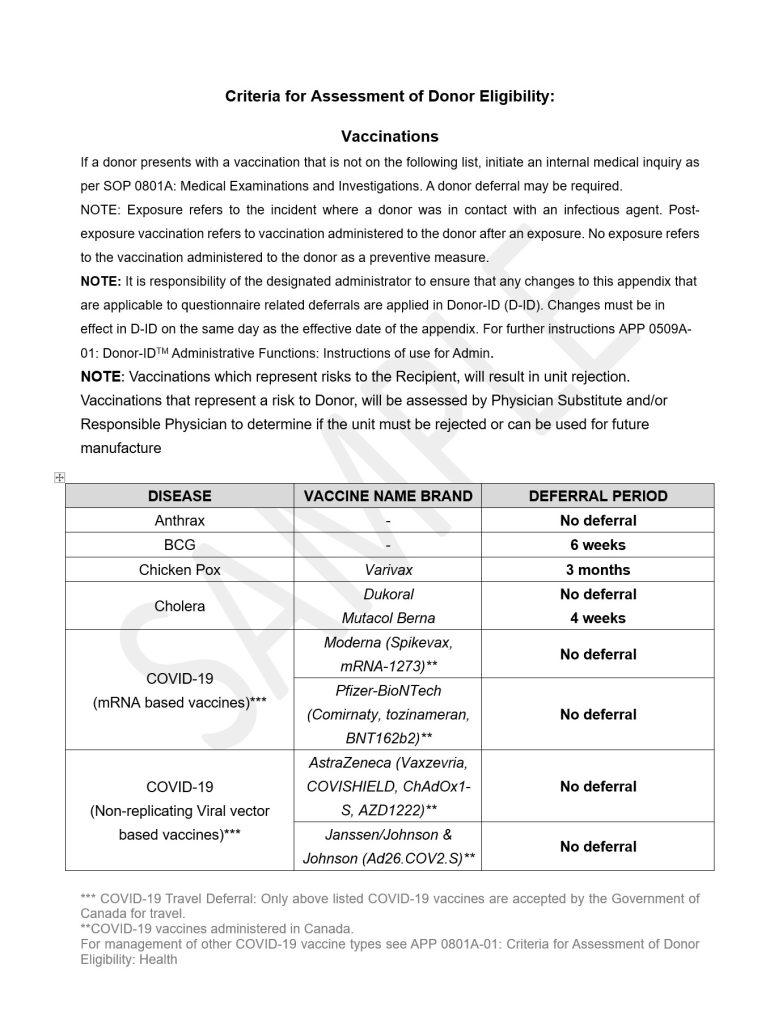
Educational Materials
Files
Links
Biologics and Genetic Therapies Directorate
Biologics and Genetic Therapies Directorate (BGTD) is the Canadian federal authority that regulates biological drugs including source plasma. BGTS also reviews establishment license applications and if compliant issues license to operate plasma centers. BGTD together with HPFBI inspect plasma centres for regulatory compliance.
Health Products and Food Branch Inspectorate (HPFBI)
Health Products and Food Branch Inspectorate (HPFBI) oversees compliance and enforcement activities. HPFBI together with BGTD conducts annual inspections for plasma centres
International Quality Plasma Program (IQPP)
International Quality Plasma Program (IQPP) is a program initiated by PPTA as part of a continuous improvement program focusing on donor management, product quality and safety.
Donating Plasma
Donatingplasma.org is an informative website sponsored by PPTA and provides valuable information on life saving plasma protein products and the need for plasma donations.
Plasma Protein Products
Canadian Blood Services (CBS), the agency charged with managing Canada’s national blood, plasma and stem cell programs in all provinces and territories across Canada, excluding Quebec. Visit the CBS for information on plasma protein products.